Today’s Dietitian
Vol. 26 No. 2 P. 20
Gastrointestinal (GI) symptoms among clients and patients are prevalent. In fact, a largescale survey has found that more than 60% of Americans from a nationally representative sample had one or more GI symptom in the past week.1 The high prevalence of GI symptoms and the significant impacts they can have on quality of life have led many consumers to turn to supplements that claim to improve digestion, including digestive enzyme supplements.
The global digestive enzyme supplement market was estimated at $699.4 million in 2021 and is projected to grow to $1.64 billion by 2031.2
Digestive enzyme supplements are designed to improve the digestion and absorption of certain food components. These products either may provide supplemental doses of enzymes naturally produced by the human body or contain other enzymes that aren’t produced in the human body but are thought to enhance the breakdown of certain foods.
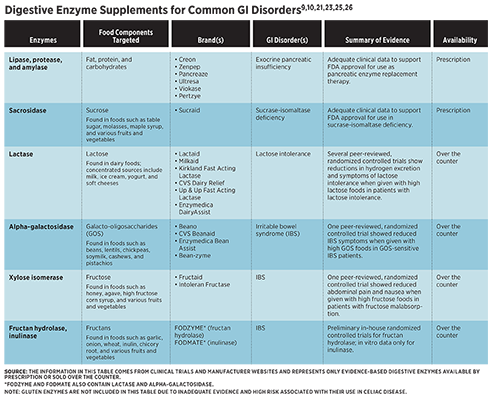
Several evidence-based digestive enzyme supplements are available that may help with gas, bloating, and altered bowel habits when used in the context of specific digestive conditions at appropriate doses. However, many digestive enzyme supplements on the market aren’t evidence based, and because over-the-counter (OTC) supplements are held to fewer rigorous safety and labeling standards than prescription medications, clients may be subject to misleading claims and exposure to contaminants when taking OTC supplements from disreputable brands.3 In addition, clients taking digestive enzyme supplements may be unclear on the recommended dosage and optimal timing. Because of this, digestive enzyme supplements are best used under the guidance of a medical provider.
This article will discuss the types of enzymes produced in the human body, the current evidence for the use of digestive enzyme supplements for various GI disorders, and counseling strategies for dietitians working with clients interested in digestive enzyme supplements.
Enzymes Naturally Present in the Body and Foods
Three categories of digestive enzymes are produced in the human body: amylases, lipases, and proteases. Amylases facilitate carbohydrate break down, starting with salivary and pancreatic amylases that convert carbohydrates into the disaccharide maltose. The enzymes maltase, lactase, and sucrase-isomaltase, found in the brush border of the small intestine, further break down maltose and the dietary disaccharides lactose and sucrose into monosaccharides for absorption. Lipases, produced in the mouth, stomach, and pancreas, break down dietary fats into monoglycerides and fatty acids. Proteases, including pepsin in the stomach, as well as trypsin, chymotrypsin, elastase, and carboxypeptidases in the pancreas, break down proteins into amino acids.4 Enzymes produced in the human body are referred to as endogenous enzymes.
Exogenous enzymes are derived from other animals, fungi, yeasts, or plants. Several digestive enzymes are naturally present in plant foods, such as amylases in mangos and bananas and lipases in avocados.5 Notably, the proteases papain in papayas, actinidin in kiwis, bromelain in pineapple, and ficin in figs may help alleviate symptoms of chronic constipation due to their association with enhanced digestion of proteins and decreased intestinal transit time.6 Exogenous enzyme supplements, available either by prescription or over the counter, may improve digestion and reduce symptoms in specific GI disorders, including exocrine pancreatic insufficiency (EPI), lactose intolerance, sucrase-isomaltase deficiency (SID), and irritable bowel syndrome (IBS).
Enzyme Supplements for GI Disorders
Exocrine Pancreatic Insufficiency
EPI is a condition in which inadequate secretion of pancreatic enzymes, particularly pancreatic lipase, leads to various symptoms, including steatorrhea (excess fat in the stools, characterized by stools that are loose, pale, greasy, oily, foul-smelling, and/or sticky), bloating, abdominal pain, fat-soluble vitamin deficiencies, and weight loss.7,8 It’s commonly associated with chronic pancreatitis, cystic fibrosis, and pancreatic cancer, and typically is diagnosed by measuring the concentration of fecal elastase via stool testing.7 The prevalence of EPI in the general population is unknown. Estimates of the prevalence of EPI in specific conditions vary widely, ranging from 30% to 90% in chronic pancreatitis, 20% to 90% in pancreatic cancers, and 80% to 90% in cystic fibrosis.7
Pancreatic enzyme replacement therapy (PERT) is the cornerstone of treatment for EPI. There are six FDA-approved prescription PERTs: Creon, Zenpep, Pancreaze, Ultresa, Viokase, and Pertzye.9 Each contains a blend of porcine-derived amylases, proteases, and lipases. Guidelines recommend that adults should start PERT at 25,000 to 50,000 lipase units per meal and 20,000 lipase units per snack. Adults can increase and adjust dosing if needed based on clinical efficacy and the amount of fat in the meal, respectively.7,9 PERT should be taken with the first bite of a meal. For meals lasting 15 to 30 minutes, taking half the dose at the beginning of the meal and half the dose in the middle of the meal may increase efficacy. For meals lasting longer than 30 minutes, patients may benefit from splitting PERT into three doses taken at the beginning, middle, and end of the meal.9
Lactose Intolerance
Lactose intolerance is caused by reduced activity of the enzyme lactase, which leads to malabsorption of lactose. This can cause symptoms of abdominal pain, bloating, diarrhea, and flatulence due to the increased osmotic load in the small intestine and the fermentation of lactose in the large intestine. Lactose intolerance is a common condition with an estimated global prevalence of 57% to 65%.10
Hydrogen breath testing, which measures exhaled hydrogen gas after consumption of lactose, is a common tool for diagnosing lactose malabsorption given that hydrogen production in the large intestine only occurs if lactose is malabsorbed in the small intestine. However, the degree of lactose malabsorption as measured by hydrogen breath testing doesn’t always correlate well with symptom severity.11 Variability in symptom severity likely is due to differences in endogenous lactase expression, microbiome composition, gut transit time, and the presence of visceral hypersensitivity in some individuals.10,1 1
In multiple studies, lactase enzyme supplements have been shown to significantly reduce breath hydrogen excretion and symptoms of lactose intolerance when administered with high lactose foods.12-16 In the studies that disclosed dosage, the amount of lactase administered ranged from 3,000 IU to 11,250 IU. Commercial products typically provide around 9,000 IU of lactase per serving through tablets, capsules, powders, liquids, or chewable forms. Lactase enzyme supplements are derived from yeast (Kluyveromyces lactis) or fungi (Aspergillus oryzae or Aspergillus niger).10 Evidence suggests that lactase from K. lactis may be more efficacious than lactase derived from A. niger.17
Moreover, adding lactase to dairy products in advance of consumption may further improve tolerance.15 Several commercially available brands that prehydrolyze their products with lactase to yield a lactose-free product are available, including Lactaid and Green Valley Organics.
Sucrase-Isomaltase Deficiency
In SID, absent or diminished activity of the enzyme sucrase-isomaltase causes malabsorption of sugar and starch. Similar to lactose intolerance, this leads to osmotic effects in the small intestine and fermentation of undigested sugars in the large intestine, resulting in GI symptoms such as diarrhea, abdominal pain, bloating, and gas.18
SID is diagnosed either through disaccharidase assays that assess the sucraseisomaltase activity of biopsies obtained from the small intestine or through breath testing.18,19 While SID previously was thought to be a rare condition, a recent systematic review found that sucrase and maltase deficiency was present in approximately 9% of pediatric patients undergoing esophagogastroduodenoscopy, as assessed by disaccharidase assays.20 There are three types of SID: congenital SID, a homozygous recessive disorder; genetic SID, a heterozygous disorder; and acquired or secondary SID, which occurs secondary to another condition such as celiac disease, inflammatory bowel disease, or small intestinal bacterial overgrowth. Secondary SID may improve or resolve when the underlying condition is treated.18 There’s an increased prevalence of sucrase-isomaltase gene variants in patients with IBS, suggesting that SID may be misdiagnosed as IBS in some cases.19
Sucraid (sacrosidase) is an FDA-approved prescription enzyme for the treatment of genetically determined sucrase deficiency.21 In a randomized, double-blinded trial, 81% of congenital SID patients were asymptomatic on an unrestricted diet with the use of sacrosidase.22 Because sacrosidase provides enzyme replacement for sucrase but not for isomaltase, some patients on sacrosidase may need to limit starch intake for further symptom management.21
The recommended dose for sacrosidase is 1 mL (8,500 IU) in patients weighing 15 kg or less and 2 mL (17,000 IU) in patients weighing more than 15 kg. Each 1 mL of sacrosidase should be mixed in 60 mL of water, milk, or infant formula. Half of the dose should be administered at the beginning of the meal, and half should be administered during the meal. To avoid enzyme degradation, sacrosidase must be refrigerated and shouldn’t be mixed with hot beverages or juices. Because it’s derived from baker’s yeast, its use isn’t recommended in patients with hypersensitivities to yeast.21
Irritable Bowel Syndrome
IBS is one of the most common GI conditions, affecting an estimated 7% to 15% of the population. IBS symptoms, which include abdominal pain, bloating, gas, and altered bowel habits, can be managed with medications, supplements, psychological treatments, and dietary modification.23 There’s emerging evidence suggesting that digestive enzyme supplements, particularly those that improve the digestion of FODMAPs, can reduce symptoms in individuals with IBS.
FODMAPs are a class of carbohydrates that are poorly absorbed in the small intestine and highly fermentable in the large intestine. There are five categories of FODMAPs: lactose, fructose, fructans, galacto-oligosaccharides (GOS), and polyols.24 As previously discussed, lactase enzyme supplements may enhance lactose absorption and reduce symptom response to the ingestion of lactose. Similarly, the digestive enzyme supplement alpha-galactosidase, which helps the breakdown of GOS, may reduce symptoms in patients with IBS. A randomized, double-blinded, placebo-controlled study found that supplementation with 300 GalU alpha-galactosidase reduced symptoms associated with intake of high-GOS foods in GOS-sensitive IBS patients.24
Xylose isomerase, fructan hydrolase, and inulinase, which are used in industrial syrup production, also may have applications for symptom management in IBS.25,26 A randomized, double-blinded, placebo-controlled study found that supplementation with 43 mg of xylose isomerase significantly reduced breath hydrogen excretion, abdominal pain, and nausea when administered with 25 g of fructose in individuals with fructose malabsorption, which was diagnosed based on breath testing. There was no significant impact on bloating.25 While this study included only individuals with positive fructose breath tests, research suggests that patients with IBS respond positively to a low-fructose diet independent of fructose breath test results.27 Xylose isomerase, therefore, may have applications for aiding fructose breakdown in a broader population of patients with IBS, though more research is needed to explore its potential benefits in IBS patients.
No peer-reviewed trials evaluating the use of fructan hydrolase or inulinase in individuals with digestive symptoms exist to date. However, in an in vitro simulation of GI digestion, inulinase has been shown to effectively break down high-fructan ingredients, including inulin and garlic, as well as a high-fructan test meal containing garlic, onion, Brussels sprouts, and black beans.28 In addition, preliminary in-house randomized, blinded, placebo-controlled trials conducted by Kiwi Biosciences demonstrated significantly reduced flatulence and diarrhea events in healthy individuals who were given supplemental fructan hydrolase powder with a highly concentrated source of fructan in chicory root bars.26 More research is needed to determine whether inulinase and fructan hydrolase are effective tools for symptom management in IBS.
All of the OTC enzymes discussed in this section—lactase, alpha-galactosidase, xylose isomerase, inulinase, and fructan hydrolase—are generally recognized as safe per the FDA.29
Celiac Disease
Celiac disease is an autoimmune disease in which an autoimmune response to gluten causes small intestinal inflammation and damage and symptoms such as diarrhea, constipation, abdominal pain, fatigue, nutrient deficiencies, and chronic headaches. Celiac disease affects an estimated 0.5% to 1% of the population and is treated with a strict gluten-free diet.30
While there are a variety of commercially available digestive enzyme supplements that make claims related to the digestion of gluten, there’s inadequate evidence to recommend them for celiac disease and other gluten-related disorders. A common ingredient in these products is the fungal enzyme DPPIV. DPPIV doesn’t fully break down immunogenic peptides and isn’t active at the low pH found in the gastric environment.31 Evidence regarding the ability of the enzymes AN-PEP and latiglutenase (formerly known as ALV003) to break down gluten into nonimmunogenic peptides in the gastric environment is more promising; however, there’s inadequate evidence to support a beneficial effect on the biomarkers and symptoms of celiac disease or other gluten-related disorders.31 Given that small amounts of gluten can produce harmful effects in celiac disease, and factors that impact efficacy of gluten-degrading enzymes aren’t fully understood, the gluten-free diet currently is the only safe and effective treatment.
Counseling Strategies for RDs
When discussing digestive enzyme supplements with clients, emphasizing that enzymes act on specific food components will help them navigate the unregulated health claims found on OTC products. Many commercially available digestive enzyme blends claim to improve general digestion without providing guidance on which foods consumers should take them with. This may lead clients to assume they can take digestive enzymes with every meal, which may or may not be the case depending on the foods they’re consuming and the foods the enzyme supplement targets. In addition, OTC products may contain enzymes that aren’t evidence based and may not include therapeutic doses of those supported by science. Providing clients with product recommendations and active ingredients and dosages to look for on OTC digestive enzyme supplements will guide them toward options that best meet their needs. Dietitians also should provide education on the foods that contain the dietary component the enzyme acts upon to better target the enzyme therapy.
For both prescription and OTC digestive enzyme supplements, it’s critical to discuss proper dosage and timing. Clients should take digestive enzyme supplements with the first bite of a meal and can consider a split dose for longer meals. Prescription enzymes should be dosed based on the manufacturer’s instructions. For clients using OTC digestive enzymes due to food intolerances, gradual reintroductions of avoided foods can help them find their threshold for tolerance with and without digestive enzyme supplements and determine what enzyme dose they need. If a digestive enzyme supplement is shown to benefit a client, strategies such as keeping supplements in an easily accessible place and setting reminders to take them will help support successful integration with the client’s dietary pattern.
As the popularity of digestive enzyme supplements continues to grow, dietitians have an important role to play in dispelling misinformation and false claims, directing clients towards evidence-based products, and counseling them on appropriate use of digestive enzyme supplements.
— Kate Evans, MS, RDN, is a clinical dietitian at the UCLA Vatche & Tamar Manoukian Division of Digestive Diseases and a consultant dietitian at Kelly Jones Nutrition, a performance nutrition private practice that supports athletes at every level.
References
1. Almario CV, Ballal ML, Chey WD, Nordstrom C, Khanna D, Spiegel BMR. Burden of gastrointestinal symptoms in the United States: results of a nationally representative survey of over 71,000 Americans. Am J Gastroenterol. 2018;113(11):1701-1710.
2. Digestive enzymes market to reach USD 1.64 billion, globally, by 2031 at 8.7% CAGR: Allied Market Research. Globe Newswire website. https://www.globenewswire.com/en/news-release/2022/08/26/2505298/0/en/Digestive-Enzymes-Market-to-Reach-USD-1-64-Billion-Globally-by-2031-at-8-7-CAGR-Allied-Market-Research.html. Published August 26, 2022. Accessed November 9, 2023.
3. Varayil JE, Bauer BA, Hurt RT. Over-the-counter enzyme supplements: what a clinician needs to know. Mayo Clin Proc. 2014;89(9):1307-1312.
4. Nelms M, Sucher K. Nutrition Therapy and Pathophysiology. Cengage Learning; 2020.
5. Raman R. 12 foods that contain natural digestive enzymes. Healthline website. https://www.healthline.com/nutrition/natural-digestive-enzymes. Published March 20, 2023. Accessed November 9, 2023.
6. Bellini M, Tonarelli S, Barracca F, et al. Chronic constipation: is a nutritional approach reasonable? Nutrients. 2021;13(10):3386.
7. Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019;12:129-139.
8. Azer SA, Sankararaman S. Steatorrhea. In: StatPearls [Internet]. StatPearls Publishing; 2023.
9. Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency — breaking the myths. BMC Med. 2017;15(1):29.
10. Catanzaro R, Sciuto M, Marotta F. Lactose intolerance: an update on its pathogenesis, diagnosis, and treatment. Nutr Res. 2021;89:23-34.
11. Ibba I, Gilli A, Boi MF, Usai P. Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting Lactose Malabsorption and Intolerance. BioMed Res Int. 2014;2014:1-7. doi:10.1155/2014/680196
12. Ojetti V, Gigate G, Gabrielli M, et al. The effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose intolerant patients: randomized trial. Eur Rev Med Pharmacol Sci. 2010;14(3):163-170.
13. Baijal R, Tandon RK. Effect of lactase on symptoms and hydrogen breath levels in lactose intolerance: a crossover placebo‐controlled study. JGH Open. 2021;5(1):143-148.
14. Lin MY, Dipalma JA, Martini MC, Gross CJ, Harlander SK, Savaiano DA. Comparative effects of exogenous lactase (beta-galactosidase) preparations on in vivo lactose digestion. Dig Dis Sci. 1993;38(11):2022-2027.
15. Montalto M, Nucera G, Santoro L, et al. Effect of exogenous β-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr. 2005;59(4):489-493.
16. Portincasa P, Di Ciaula A, Vacca M, Montelli R, Wang DQ‐H, Palasciano G. Beneficial effects of oral tilactase on patients with hypolactasia. Eur J Clin Invest. 2008;38(11):835-844.
17. Ianiro G, Pecere S, Giorgio V, Gasbarrini A, Cammarota G. Digestive enzyme supplementation in gastrointestinal diseases. Curr Drug Metab. 2016;17(2):187-193.
18. Cohen SA. The clinical consequences of sucrase-isomaltase deficiency. Mol Cell Pediatr. 2016;3(1):5.
19. Chey WD, Cash B, Lembo A, Patel D, Scarlata K. Congenital sucrase-isomaltase deficiency: what, when, and how? Gastroenterology & Hepatology. 2020;16(10):Suppl 5.
20. Daileda T, Baek P, Sutter ME, Thakkar K. Disaccharidase activity in children undergoing esophagogastroduodenoscopy: a systematic review. World J Gastrointest Pharmacol Ther. 2016;7(2):283.
21. Sucraid (sacrosidase) oral solution. US Food & Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020772Orig1s027Lbl.pdf. Published May 26, 2022. Accessed November 9, 2023.
22. Treem WR, McAdams L, Stanford L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr. 1999;28(2):137-142.
23. Tuck CJ, Taylor KM, Gibson PR, Barrett JS, Muir JG. Increasing symptoms in irritable bowel symptoms with ingestion of galacto-oligosaccharides are mitigated by α-galactosidase treatment. Am J Gastroenterol. 2018;113(1):124-134.
24. Tuck C, Barrett J. Re-challenging FODMAPs: the low FODMAP diet phase two. J Gastroenterol Hepatol. 2017;32 Suppl 1:11-15.
25. Komericki P, Akkilic‐Materna M, Strimitzer T, Weyermair K, Hammer HF, Aberer W. Oral xylose isomerase decreases breath hydrogen excretion and improves gastrointestinal symptoms in fructose malabsorption – a double‐blind, placebo‐controlled study. Aliment Pharmacol Ther. 2012;36(10):980-987.
26. FODZYME® clinical brief. FODZYME website. https://care.fodzyme.com/en/articles/6672080-fodzyme-clinical-brief. Accessed November 9, 2023.
27. Melchior C, Desprez C, Houivet E, et al. Is abnormal 25 g fructose breath test a predictor of symptomatic response to a low fructose diet in irritable bowel syndrome? Clin Nutr. 2020;39(4):1155-1160.
28. Guice JL, Hollins MD, Farmar JG, Tinker KM, Garvey SM. Microbial inulinase promotes fructan hydrolysis under simulated gastric conditions. Front Nutr. 2023;10:1129329.
29. Enzyme preparations used in food (partial list). U.S. Food & Drug Administration website. https://www.fda.gov/food/generally-recognized-safe-gras/enzyme-preparations-used-food-partial-list. Published January 4, 2018. Accessed October 30, 2023.
30. Posner EB, Haseeb M. Celiac disease. In: StatPearls. StatPearls Publishing; 2023.31. König J, Brummer RJ. Is an enzyme supplement for celiac disease finally on the cards? Expert Rev Gastroenterol Hepatol. 2018;12(6):531-533.



