 Insulin Delivery Device Technology — Today and Into the Future
Insulin Delivery Device Technology — Today and Into the Future
By Hope Warshaw, MMSc, RD, CDCES, BC-ADM, FADCES
Today’s Dietitian
Vol. 24 No. 8 P. 22
Learn about newer and more advanced technologies and how they can optimize outcomes and ease self-care challenges.
Today, nearly 8 million people with diabetes in the United States require insulin to manage their glucose levels. All people with type 1 diabetes, about 1.6 million, require insulin, and the majority use multiple daily injections.1 Roughly 30% to 40% of this population use an insulin pump or pod management system.2
Almost 6 million people with type 2 diabetes require insulin to manage their glucose levels. A small yet growing percentage of these individuals use a technology-enabled insulin delivery device, such as those Today’s Dietitian explores in this article.2
Most people who take insulin continue to use a traditional insulin pen or vial and syringe despite an expanding array of technology-enabled delivery devices. These devices, albeit quite varied in their capabilities, including connectivity and technological sophistication, can ease the burdens and challenges of taking insulin.3 (For more information on this topic, read the article “Insulin Delivery Devices in 2020 and Beyond” in the May 2020 issue of Today’s Dietitian). In addition, evidence shows use of some of these devices improves diabetes outcomes, specifically what’s been coined the management trifecta—lower A1c, less hypoglycemia, and higher Time in Range.4,5 (See “From A1c to Time in Range: New Glycemic Management Metrics,” in our November 2022 E-Newsletter.)
Low Usage of the Technology
There are many reasons for low usage of technology-enabled insulin delivery devices, several of which rest with the people with diabetes. They have a lack of awareness and knowledge of the devices, insufficient insurance coverage, an attitude of “if it ain’t broke, don’t fix it,” an unwillingness to allocate the time and energy involved to master a new device, or are reluctant to wear a device, such as an insulin pump.
Other reasons rest with clinicians, such as the time and energy needed to learn about new devices, the ability to work through coverage hurdles, allotting time to introduce people to devices, as well as onboarding and optimizing the device.
“It’s important for RDs to be knowledgeable about these newer insulin delivery devices to advise people about their options and help them choose and use the right technology for their needs at the time,” says Laura Russell, MA, RDN, LD, CDCES, program coordinator at the Endocrinology Clinic of Minneapolis and chair-elect of the Diabetes Dietetic Practice Group of the Academy of Nutrition and Dietetics. “Possessing the ability to confidently discuss these devices with clients helps enhance our practice,” Russell says.
Think ICC Framework
To assist clients in choosing and optimizing technology-enabled devices, the Association of Diabetes Care and Education Specialists, along with content experts, created the ICC Framework.6 The Identify, Configure and Collaborate Framework (see illustration below) is a model health care providers can use to raise clients’ awareness about available insulin delivery devices. Here’s an explanation of the framework:
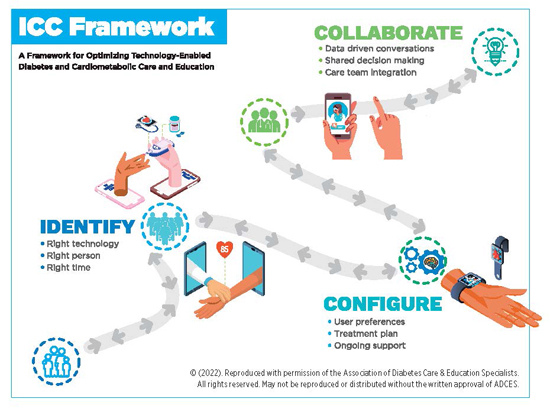
• Identify focuses on helping clients choose the right device to meet their current needs. Think about the most significant barriers clients face at the time they take insulin according to their diabetes care plan. As they go through various ages and stages with diabetes, a different device may suit them better. In addition, insulin delivery devices will continue to evolve.
• Configure focuses on helping people set up their device based on their lifestyle, personal preferences, and diabetes care goals and plan. Configure also integrates ongoing support.
• Collaborate refers to the collaborative partnership between the person with diabetes and his or her clinician to obtain and analyze device and management data to improve and advance care.
“I use the ICC Framework to introduce clients to a new technology and let them know what it does and doesn’t do compared with how they currently deliver insulin,” says Natalie J. Bellini, DNP, FNP-BC, BC-ADM, CDCES, an endocrine nurse practitioner at R&B Medical Group in Williamsville, New York. “Then we configure the device based on collaboration and may, for example, initiate a device with higher target glucose levels if the person expresses a fear of hypoglycemia,” Bellini says.
An essential message about integrating a new insulin delivery device into a person’s care plan is succinctly stated by the American Diabetes Association: “No device used in diabetes management works optimally without education, training, and follow up.”4
Connectivity Equals Communication
The entrance and availability of continuous glucose monitors (CGM) and monitoring nearly two decades ago have been revolutionary in advancing diabetes care.7 “It used to be that we would detect high or low glucose data points and had to guess the causes and solutions. Now we, along with our clients, can establish cause-and-effect relationships with a high degree of certainty,” says Gary Scheiner, MS, CDCES, owner of Integrated Diabetes Services, author of Think Like a Pancreas, and a person with diabetes for 37 years who’s worn a wide variety of diabetes management technology.
Connectivity overcomes numerous hurdles. It allows for the transfer of complete and accurate diabetes-related data (eg, glucose, insulin doses, food intake, and related timing) in understandable and usable formats for people with diabetes and their clinicians. These reports, which users typically can email, fax, or upload to their clinicians, can be analyzed to more easily assess and evolve the person’s diabetes management. Clinicians can use these reports in person or via telehealth.
Connectivity also allows communication and integration between a CGM and some insulin delivery devices. This capability has led to several automated insulin delivery (AID) systems.8
Today and Tomorrow’s Devices in the United States
What follows are key details of FDA-cleared or FDA-approved insulin delivery devices available in the United States. (For more information on FDA review processes, read “Insulin Delivery Devices in 2020 and Beyond” in the May issue of Today’s Dietitian.) Devices available only outside the United States and Do It Yourself (DIY) systems (see sidebar below) aren’t included.
DEVICE CATEGORY: Inhaled Insulin
Manufacturer: MannKind
Website: www.mannkindcorp.com
Product name: Afrezza
Description: These are prefilled single-use/dose cartridges of ultra-rapid-acting inhaled insulin in 4U, 8U, and 12U,* placed in an inhaler device. It’s used for mealtime doses (bolus) and in combination with basal (long-acting) insulin in type 1 diabetes.
FDA status and ages for intended use: Afrezza was approved as a new drug in 2014 for individuals aged 18 and older who take insulin. It’s not indicated in people with COPD and asthma** or in those who smoke.
Assets: Data integration/management platform/app: BluHale®, a bluetooth-connected accessory that attaches to the inhaler to transfer real-time data on dosing. Future data integration with CGM and third-party apps.
*Units of Afrezza aren’t equivalent to units of U100 insulin. Afrezza reaches maximum concentration in 15 minutes, maximum effect in 50 minutes, and is cleared from the body in 1.5 to 2 hours.
**Get baseline FEV1 (forced expiratory volume in 1 sec) test before starting.
DEVICE CATEGORY: Simple Patch
Manufacturer: CeQur
Website: www.cequr.com
Product name: CeQur Simplicity (Nationwide release is end of 2022.)
Description: This is a three-day wearable, disposable patch that removes mealtime insulin barriers. It provides a convenient, discreet, injection-free way to take mealtime and correction boluses. Each patch holds up to 200 units of rapid-acting insulin and delivers 2U per click.
FDA status and ages for intended use: The patch was FDA-cleared in 2010 for adults aged 21 and older who take Humalog or Novolog insulin.
Assets: Convenient, discreet, injection-free dosing with built-in safety mechanisms.
Data integration/management platform/app: None
DEVICE CATEGORY: Simple Patch
Manufacturer: Mannkind Corporation
Website: www.go-vgo.com
Product name: V-Go wearable insulin delivery (patch)
Description: Simplifies multiple daily injection (basal/bolus) therapy because it delivers both. Users fill V-Go with rapid-acting insulin (cleared for use with Humalog or NovoLog). The patch is available in 20U, 30U, or 40U for continuous basal dosing, plus up to 36U for bolus on-demand dosing in 2U increments. The patch is replaced every 24 hours.
FDA status and ages for intended use: FDA-cleared in 2011 for adults aged 21 and older who require insulin.
Assets: The patch provides discreet insulin delivery that eliminates multiple injections daily.
Data integration/management platform/app: None
DEVICE CATEGORY: Connected Device
Manufacturer: Bigfoot Biomedical
Website: www.bigfootbiomedical.com
Product name: Bigfoot Unity Diabetes Management Program
Description: Three patient-facing components include two connected smart pen caps. The pen cap fits on disposable insulin pens, one for basal and one for bolus delivery. Two other components include a mobile app and integrated Abbott FreeStyle Libre2 CGM. The system continuously monitors glucose data and displays dose recommendations from the person’s clinician found on the pen cap screen. Support is provided with reminders and real-time glucose alerts. The system is integrated with a cloud-based platform that connects users with their clinicians. (Clinicians use Remote Patient Monitoring billing codes to bill for ongoing follow up.)
FDA status and ages for intended use: FDA-cleared for people aged 12 and older with type 1 or type 2 diabetes using multiple daily injections.
Assets: All components, including the device, software, and services from clinicians, are purchased from one entity. Training and support are provided. (This system can’t be purchased individually.)
Data integration/management platform/app: The pen cap transmits CGM and insulin dose data via Bluetooth for upload to the cloud-based Bigfoot Clinic Hub platform. The mobile app allows data viewing and sharing.
DEVICE CATEGORY: Connected Device
Manufacturer: Medtronic Diabetes
Website: www.medtronicdiabetes.com/products/inpen-smart-insulin-pen-system
Product name: InPen Smart Insulin Pen
Description: This device is a durable (reusable) pen that uses prefilled 300U cartridges of rapid-acting insulin. (It’s FDA-cleared for Fiasp, Humalog, or NovoLog). The pen delivers bolus doses from 0.5U increments up to 30U.
FDA status and ages for intended use: The pen was FDA-cleared in 2016 for people aged 12 and older. In 2020, the FDA expanded clearance for all ages. (Children under age 7 require adult supervision.) In 2020, the FDA also cleared InPen for two meal modes in addition to the original carbohydrate counting.
Assets: InPen reminds users of missed doses (bolus and basal). It has auto-prime detection, a dose calculator based on individualized therapy settings similar to an insulin pump, and it records all diabetes care data.
Data integration/management platform/app: InPen has wireless transmission via Bluetooth to the InPen app, called InPen Insights. The reports from the app can be shared with clinicians. InPen integrates with Medtronic Guardian Connect CGM in real-time and Dexcom G6 CGM with a three-hour delay. InPen also connects via Apple Healthkit to blood glucose monitors and third-party apps.
DEVICE CATEGORY: Traditional Insulin Pump/Pod (No CGM Connectivity)
Manufacturer: Insulet Corporation
Website: www.omnipod.com/hcp/products/omnipod-dash-system
Product name: Omnipod DASH Insulin Management System
Description: Insulin is delivered through a filled pod (up to 200U) with automatic insertion. This is a tubeless system. Communication with the pod is through a locked-down Android device.
FDA status and ages for intended use: The system was FDA-cleared in 2018 for all people with diabetes who use insulin.
Assets: The pod is waterproof and provides discreet insulin delivery. For health plan coverage, the system isn’t considered durable medical equipment but rather a pharmacy benefit. Supplies are available through a pharmacy. The system is covered under Medicare Part D and has a lower upfront cost.
Data integration/management platform/app: Bluetooth wireless technology. Two apps, Omnipod DISPLAY (for users) and VIEW (for users to share data with up to 12 people), enable data sharing through Glooko, and an iPhone widget allows viewing of insulin and CGM data (Dexcom).
DEVICE CATEGORY: Insulin Pumps and Pod (Hybrid Closed Loop [HCL], Advanced Hybrid Closed Loop [AHCL], and AID Systems)
Manufacturer: Insulet Corporation
Website: www.omnipod.com/hcp/products/omnipod5
Product name: Omnipod 5 AID System
Description: This is a fully on-body AHCL/AID system. The Personalized Model Predictive Control algorithm is integrated with the pods. Users can customize a target blood glucose (from 110 mg/dL to 150 mg/dL in 10 mg/dL increments) that’s adjustable by time of day. Users still must enter the amount of carbohydrates they consume into the SmartBolus calculator.
FDA status and ages for intended use: This system was FDA-cleared in early 2022 for people with type 1 diabetes aged 6 and older. It was FDA-cleared for individuals aged 2 and older in mid-2022.
Assets: The system has an activity feature for decreased insulin needs (eg, exercise). It includes waterproof pods and discreet insulin delivery. The controller or compatible smartphone doesn’t need to be near a pod for automated basal insulin delivery in manual mode and micro boluses in automated mode.
Data integration/management platform/app: The Dexcom G6 transmitter communicates directly to the pod. The pod communicates wirelessly to the Omnipod 5 app on the controller or compatible smartphone, which controls and monitors the pod operations via Bluetooth technology.
DEVICE CATEGORY: Insulin Pumps and Pod
Manufacturer: Medtronic Diabetes
Website: www.medtronicdiabetes.com
Product name: MiniMed 770G
Description: The MiniMed 770G is an HCL device that functions with the SmartGuard™ Auto Mode algorithm. The device requires bolus doses for food/carbohydrate. It automatically adjusts basal insulin every five minutes based on integrated CGM (Guardian™ Sensor 3) results. It helps prevent hyperglycemia and hypoglycemia and suspends insulin up to 30 minutes before reaching the preset low glucose level and automatically restarts when the glucose level recovers. In addition, it holds up to 300U of rapid-acting insulin. It comes with the Roche Accu-Chek Guide Link BGM that connects wirelessly.
FDA status and ages for intended use: The device was FDA-approved in 2020 for people with type 1 diabetes aged 2 and older.
Assets: The pump is waterproof, holds 90 days of history, and delivers insulin in low increments beneficial for small children.
Data integration/management platform/app: Carelink software integrates all the data. Smartphone connectivity enables people to view pump and CGM data and receive device notifications on their phone. The device also offers data sharing, and Bluetooth connectivity allows for future software updates (see MiniMed 780G).
DEVICE CATEGORY: Insulin Pumps and Pod
Manufacturer: Tandem Diabetes Care
Website: www.tandemdiabetes.com
Product name: t:slim X2 with Control-IQ (t:slim X2 also is available alone or with basal IQ.)
Description: This is an AHCL/AID insulin pump system. It predicts and helps prevent hypo- and hyperglycemia and connects with CGM (Dexcom G6) data to hold glucose levels in the range of 70 mg/dL to 180 mg/dL. The system provides autocorrect bolus doses and offers optional settings for sleep and exercise.
FDA status and ages for intended use: The system was FDA-cleared in 2019 as an interoperable automated glycemic controller (iAGC) device for people aged 14 and older. It was FDA-cleared in 2020 for individuals aged 6 and older.
Assets: It provides free updatable software uploads for next-generation software with a prescription. Training and online tutorials are required.
Data integration/management platform/app: The system is integrated with Dexcom G6 CGM. Individuals should use t:connect to access pump data and settings and upload pump data at clinician visits. Bolus doses now can be delivered using the t:connect mobile app and a compatible smartphone, app, and software update. Training is required.
Future: Mobi (t:sport) is Tandem’s next generation device. Mobi will be a discreet tubed micropump with no screen. It contains an AID algorithm controlled via the user’s mobile phone. Timing: Tandem is in the final stages of testing Mobi and plans to complete its 510(k) application for FDA clearance by end of 2022.
DEVICE CATEGORY: Future Insulin Pumps and Pod
Currently Under FDA Review
Developer: Beta Bionics (a public benefit company & B Corp)
Website: www.betabionics.com
Product name: iLet® Bionic Pancreas* (insulin-only)9
Description: iLet is a pocket-sized, wearable device designed to autonomously dose insulin. Users enter only body weight to initialize therapy. The iLet automatically titrates and infuses insulin doses without user inputs and parameters, and carbohydrate counting isn’t required. Users make a meal announcement when they eat. This is based on a relative carbohydrate intake for the person. The system keeps learning and adjusting based on the wearer’s behaviors and glucose results.
FDA status and ages for intended use: Beta Bionics submitted the iLet for FDA clearance in mid-2022 through the Breakthrough Device Designation pathway.
Future: The iLet® Duo will be bihormonal, using both insulin and glucagon. The system will be integrated with the Dexcom G6.9
*Caution: The iLet bionic pancreas is an investigational device limited by Federal (or United States) law to investigational use. Not available for sale.
DEVICE CATEGORY: iOS App for DIY Looping
Developer: Tidepool, a nonprofit entity. The goal is to build and support an FDA-regulated version of Loop for automated insulin dosing. (Tidepool Loop is not cleared for use in the US or outside of the US. Displayed is a conceptual rendering of a product in development.)
Website: www.tidepool.org/automated-insulin-dosing
Product name: Tidepool Loop isn’t a standalone AID system. The goal is to make software interoperable with alternate controller-enabled pumps and integrated CGMs. Announced partners: Medtronic, Insulet, and Dexcom.
Description: Tidepool Loop intends to be delivered as an iOS app available in the Apple App Store with a prescription.
FDA status and ages for intended use: The app has been submitted to FDA as an iAGC and is currently under review.
Assets: The app has interoperability with other alternate controller-enabled pumps and integrated CGMs.
Data integration/management platform/app: To be determined
DEVICE CATEGORY: Future Insulin Pumps and Pod
Manufacturer: Medtronic
Website: www.medtronic-diabetes.co.uk (UK website, not FDA-approved to date)
Product name: MiniMed 780G system with Smart-Guard™ technology
Description: The system is AHCL/AID. The SmartGuard™ algorithm automates insulin delivery in a real-time adaptable way with predictive personalized diagnostics.
FDA status and ages for intended use: The 780G was submitted for FDA-approval in April 2021. The system received the CE mark in Europe in 2020 and is now available globally in 60-plus countries.
Assets: Using meal detection technology, the 780G automatically adjusts background insulin with correction boluses every five minutes based on integrated CGM results. It allows for adjustable target settings and assists with missed bolus forgiveness as well as late dosing for meals or underestimating the amount of carbohydrates consumed.
Data integration/management platform/app: Carelink software integrates all data. Smartphone connectivity allows viewing of pump and CGM information and device notifications on the phone. It also offers remote access to data for care partners. Bluetooth connectivity will allow for future software updates. Once this device is FDA-approved, people currently using the 770G will be eligible for a no-cost software upgrade without the need to swap their pump for a new model.
Future: Plans are in the works to introduce a personalized closed loop insulin pump system that will be more advanced than the 780G. The system has FDA Breakthrough Devices Designation and is currently in feasibility trials.
Glimpse of the Future
“Many brilliant people, manufacturers, and related entities are pushing full steam ahead to close the insulin delivery loop to reduce the burdens of managing insulin-requiring diabetes,” Bellini says. Beyond devices, there’s work on faster-acting insulins that will more quickly blunt the rise of postprandial glucose levels. Conversely, there’s work on once-weekly basal insulin, likely for greater use in type 2 diabetes. In addition, myriad efforts are being researched and/or integrated into devices to reduce the burdens of carbohydrate counting and prebolus dosing in sync with food intake. Along this line, a general trend is to make insulin delivery devices smarter by integrating artificial intelligence, machine learning, predictive analytics, and/or personalized precision insulin dosing.
These are, indeed, exciting times in insulin delivery technology.
— Hope Warshaw, MMSc, RD, CDCES, BC-ADM, FADCES, is owner of Hope Warshaw Associates, LLC, a diabetes- and nutrition-focused consultancy based in Asheville, North Carolina. She’s a book author and freelance writer specializing in diabetes care. She trains on several technologies and provides diabetes counseling through 9amhealth. Warshaw served as the 2016 president of the Association of Diabetes Care and Education Specialists and currently serves as the 2022–2023 chair of the Academy of Nutrition and Dietetics Foundation.
References
1. Type 1 diabetes facts. JDRF website. https://www.jdrf.org/t1d-resources/about/facts/. Accessed September 6, 2022.
2. Sikes KA, Weyman K. Diabetes and the use of insulin pumps. Endocrinology Advisor website. https://www.endocrinologyadvisor.com/home/decision-support-in-medicine/endocrinology-metabolism/diabetes-and-the-use-of-insulin-pumps/. Accessed September 6, 2022.
3. Warshaw H, Isaacs D, MacLeod J. The reference guide to integrate smart insulin pens into data-driven diabetes care and education services. Diabetes Educ. 2020;46(Supp 4):3S-20S.
4. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 7. Diabetes technology: standards of medical care in diabete– - 2022. Diabetes Care. 2022;45(Supp 1):S97-S112.
5. Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology clinical practice guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocrine Prac. 2021;27(6):505-537.
6. Greenwood DA, Howell F, Scher L, et al. A framework for optimizing technology-enabled diabetes and cardiometabolic care and education: the role of the diabetes care and education specialist. Diabetes Educ. 2020;46(4):315-322.
7. Didyuk O, Econom N, Guardia A, Livingston K, Klueh U. Continuous glucose monitoring devices: past, present, and future focus on the history and evolution of technological innovation. J Diabetes Sci Technol. 2021;15(3):676-683.
8. Phillip M, Nimri R, Bergenstal RM, et al Consensus recommendations for the use of automated insulin delivery (AID) technologies in clinical practice [published online September 6, 2022]. Endocr Rev. doi: 10.1210/endrev/bnac022.
9. Warshaw H. Latest news on Beta Bionics’s iLe– - interview with interim CEO, Martha Goldberg Aronson. T1D website. https://t1dexchange.org/latest-news-on-beta-bionicss-ilet-interview-with-interim-ceo-martha-goldberg-aronson/. Accessed September 6, 2022.
Terms & Language Defined
Hybrid closed loop (HCL): A system is considered “hybrid” because it doesn’t fully close the communication loop as it delivers insulin. Users still must consider food intake, choose bolus insulin doses, and deliver the doses.
Advanced hybrid closed loop (AHCL): AHCL systems have similar capabilities as HCL systems. Some entities use this term to define their automated insulin delivery (AID) system.
AID: The FDA has used this term to describe HCL and AHCL systems that aren’t fully closed loop.
Currently, there’s no insulin pump management system that fully closes the insulin delivery loop. However, systems are inching closer. A fully closed loop system wouldn’t require user input of information to increase or decrease insulin delivery. Insulin delivery would be fully responsive to real-time glucose data and keep glucose levels within the person’s set goals 24/7.
The following interrelated elements are integral to how HCL, AHCL, and AID systems work.
Interoperable automated glycemic controller (iAGC): This device automatically adjusts insulin delivery by connecting to an alternate controller-enabled (ACE) pump.
ACE pump: This automatically can adjust insulin delivery through the use of an iAGC.
Integrated continuous glucose monitor (iCGM): This is a type of continuous glucose monitor that’s integrated with other compatible devices and electronic interfaces, such as an AID dosing system.
— HW
Do-It-Yourself Automated Insulin Delivery Systems Today
The do-it-yourself (DIY) automated insulin delivery community is growing strong globally and is estimated to be greater than 25,000 users.1-3 Interestingly, even with today’s connected insulin pump/pod management systems, some people still choose to use a non–FDA-reviewed DIY automated insulin delivery system. “The DIY systems allow significantly more customization and fine-tuning than the commercially available systems, which must put many restrictions/guardrails [in place] in order to receive FDA clearance,” says Gary Scheiner, MS, CDCES, owner of Integrated Diabetes Services, author of Think Like a Pancreas, and a person with diabetes for 37 years. “And from what I have seen (and experienced personally), for people who have good self-management skills and like to remain actively engaged in their day-to-day care, the DIY systems tend to produce superior glycemic management.”
— HW
References
1. Braune K, Lal RA, Petruželková L, et al. Open-source automated insulin delivery: international consensus statement and practical guidance for health-care professionals. Lancet Diabetes Endocrinol. 2022;10(1):58-74.
2. Lewis DM. Do-it-yourself artificial pancreas system and the OpenAPS movement. Endocrinol Metab Clin North Am. 2020;49(1):203-213.
3. Lal R, Buckingham B. Open source automated insulin delivery (OS-AID): debate. Paper presented at: Association of Diabetes Care and Education Specialists 2022 annual conference.
Stay Abreast
The following tips will help dietitians stay current on the nonstop evolution of insulin delivery technology.
• “Be a member of the Diabetes DPG to access newsletters, e-news, the discussion board, webinars, and gain access to collegial dialog,” says Laura Russell, MA, RDN, LD, CDCES, program coordinator at the Endocrinology Clinic of Minneapolis and chair-elect of the Diabetes Dietetic Practice Group of the Academy of Nutrition and Dietetics.
• Become a member of the Association of Diabetes Care and Education Specialists, and join the technology-focused community of interest.
• Befriend the manufacturers’ sales and clinical representatives. They know the most about their products and what’s in the pipeline.
• Read technology-focused journals, specifically Diabetes Technology and Therapeutics and Journal of Diabetes Science and Technology.
Access the following free internet-based reliable resources:
• The Association of Diabetes Care and Education Specialists’ danatech (www.danatech.org) is now accessible by anyone at no cost. The recently revamped danatech is a go-to resource on diabetes technologies, along with opportunities to earn CE and certificates.
• DiabetesWise was developed by experts at Stanford University with support from the Helmsley Charitable Trust. They’ve featured a consumer side (https://diabeteswise.org) for several years and recently launched a provider side (https://providers.diabeteswise.org/#/devices/device-library), where clinicians can learn about insulin delivery devices and continuous glucose monitors.
• Panther Diabetes Technology (www.pantherprogram.org), which was developed by experts at the Barbara Davis Diabetes Center, University of Colorado, offers information about its C.A.R.E.S. Framework and point-of-care clinical tools, and provides insulin delivery device info sheets along with device comparison charts.
— HW